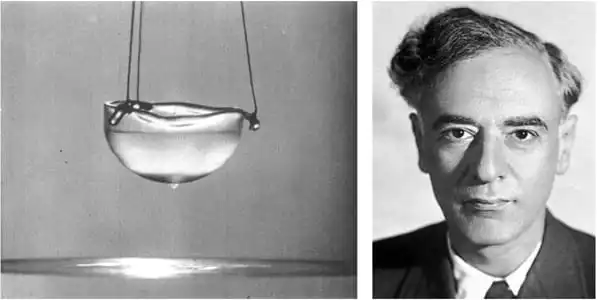
On July 10, 1908, helium was liquefied for the first time ever. It was achieved by Dutch physicist Heike Kamerlingh Onnes, who won the Nobel Prize in Physics in 1913 for his low temperature work that led to the production of liquid helium.
Helium is a chemical element that has the lowest boiling and melting points among all the elements. The first in the noble gas group in the periodic table, helium is a colourless, odourless, non-toxic, inert gas in nature. Liquid helium was produced for the first time only in 1908, thanks to the work done by Dutch physicist Heike Kamerlingh Onnes,
Born in Groningen, The Netherlands in 1853, Kamerlingh Onnes father was the owner of a brickworks and his mother was the daughter of an architect. He received additional teaching in Greek and Latin after spending his time in a secondary school without classical languages in his native town.
Displays his talents early
From 1871-73, Kamerlingh Onnes went to Heidelberg University as a student of German physicists Robert Bunsen and Gustav Kirchhoff. At the age of 18, his talents in the scientific field were apparent as he was awarded a Gold Medal for a competition sponsored by the Natural Sciences Faculty at the University of Utrecht and followed it up with a Silver Medal in a similar event at the University of Groningen the next year.
He was awarded his doctorate by the University of Groningen in 1879 with a remarkable thesis ‘Nieuwe bewijzen voor de aswenteling der aarde’ (New proofs of the rotation of the Earth). After teaching at the Polytechnic School in Delft until 1882, he was appointed to the Physics Chair at the University of Leiden, where he served as a professor until 1923.
Coldest spot on Earth
Inspired by the theories and works of his compatriots Johannes van der Waals and Hendrik Lorentz Kamerlingh Onnes reorganised the Physical Laboratory at Leiden and built up the Cryogenic Laboratory that now bears his name in order to suit his own programme. This meant that Leiden soon established itself as the low-temperature research centre of the world, with some going to the extent of saying that the coldest spot on Earth was situated at Leiden
He spent over a decade perfecting cryogenic experimental techniques, while also studying metals and fluids at low temperatures, Having succeeded in building an improved hydrogen liquefaction machine by 1906, his efforts adminated in the production of liquid helium on July 10, 1908.
On that wet and windy day, Kamerlingh Onnes woke before dawn and headed to his laboratory in the centre of the town, where technicians were already hard at work. Having already increased the stock of liquid air to 75 litres the previous day, they went about the first task of liquefying hydrogen. By 1.30pm, they had produced the 20 litres of liquid hydrogen necessary to launch the attack on helium and stored it in Dewar flasks.
Based on theory, Kamerlingh Onnes knew how much hydrogen they needed and the amount of time the helium experiment would take. It was time to start cooling the helium at 2.30pm, and in just another half an hour, the temperature had already fallen to 93 Kelvin (-180 degree Celsius).
Iterative technique
Kamerlingh Onnes employed the same iterative technique that had allowed their laboratory to produce liquid hydrogen at the increased rate of 4 litres per hour in 1906. This meant that the helium gas that was pre-cooled by liquid hydrogen and liquid air was allowed to expand through a porous plug, thereby cooling to even lower temperatures. This is then recirculated back to the other side of the plug where the expanded helium is further cooled by expanding through the plug again.
By 6.30pm, the temperatures were lower than that of liquid hydrogen and eventually reached 6 Kelvin (-267 degree Celsius). Down to the last flask of liquid hydrogen, Kamerlingh Onnes attached it to the apparatus and the team was wondering if they were destined for failure as the helium had already circulated 20 times with nothing to show for it.
Small teacup of helium
The temperature stabilised at 4 Kelvin (-269 degree Celsius) by 7.30pm when a colleague who came to see how the experiment was going remarked that the thermometer appeared to be standing in a bath of liquid. On closer inspection, Kamerlingh Onnes was able to make out the liquid surface of liquid helium! The experiment had produced just a small teacup of liquid helium, about 60ml to be precise.
Kamerlingh Onnes also discovered and coined the term superconductivity in 1911, demonstrating that the resistance of certain electrical conductors totally disappeared suddenly at a temperature near absolute zero (-273 degree Celsius). The low-temperature studies that resulted in the liquefaction of helium in 1908 helped him win the Nobel Prize in Physics in 1913, 13 years before his death at Leiden in 1926.
Picture Credit : Google