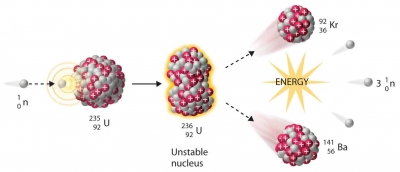
An atom is the building block of all matter. Nuclear energy is the energy that exists at the core of the atom called the nucleus. Nuclear energy can be accessed through two kinds of atomic reactions nuclear fission and nuclear fusion. In the first reaction, atoms are broken apart whereas in the latter they are forced to fuse together. However, to date, nuclear power plants do not have a safe and reliable way to generate energy through nuclear fusion. Therefore, nuclear reactors use uranium as fuel to produce energy by nuclear fission.
World nuclear power
Nuclear power provides almost 15 percent of the world’s electricity. The first nuclear power plants, which were small demonstration facilities, were built in the 1960s. These prototypes provided “proof-of-concept” and laid the groundwork for the development of the higher-power reactors that followed.
The nuclear power industry went through a period of remarkable growth until about 1990, when the portion of electricity generated by nuclear power reached a high of 17 percent. That percentage remained stable through the 1990s and began to decline slowly around the turn of the 21st century, primarily because of the fact that total electricity generation grew faster than electricity from nuclear power while other sources of energy (particularly coal and natural gas) were able to grow more quickly to meet the rising demand. This trend appears likely to continue well into the 21st century. The Energy Information Administration (EIA), a statistical arm of the U.S. Department of Energy, has projected that world electricity generation between 2005 and 2035 will roughly double (from more than 15,000 terawatt-hours to 35,000 terawatt-hours) and that generation from all energy sources except petroleum will continue to grow.
In 2012 more than 400 nuclear reactors were in operation in 30 countries around the world, and more than 60 were under construction. The United States has the largest nuclear power industry, with more than 100 reactors; it is followed by France, which has more than 50. Of the top 15 electricity-producing countries in the world, all but two, Italy and Australia, utilize nuclear power to generate some of their electricity. The overwhelming majority of nuclear reactor generating capacity is concentrated in North America, Europe, and Asia. The early period of the nuclear power industry was dominated by North America (the United States and Canada), but in the 1980s that lead was overtaken by Europe. The EIA projects that Asia will have the largest nuclear capacity by 2035, mainly because of an ambitious building program in China.
A typical nuclear power plant has a generating capacity of approximately one gigawatt (GW; one billion watts) of electricity. At this capacity, a power plant that operates about 90 percent of the time (the U.S. industry average) will generate about eight terawatt-hours of electricity per year. The predominant types of power reactors are pressurized water reactors (PWRs) and boiling water reactors (BWRs), both of which are categorized as light water reactors (LWRs) because they use ordinary (light) water as a moderator and coolant. LWRs make up more than 80 percent of the world’s nuclear reactors, and more than three-quarters of the LWRs are PWRs.
Issues affecting nuclear power
Countries may have a number of motives for deploying nuclear power plants, including a lack of indigenous energy resources, a desire for energy independence, and a goal to limit greenhouse gas emissions by using a carbon-free source of electricity. The benefits of applying nuclear power to these needs are substantial, but they are tempered by a number of issues that need to be considered, including the safety of nuclear reactors, their cost, the disposal of radioactive waste, and a potential for the nuclear fuel cycle to be diverted to the development of nuclear weapons. All of these concerns are discussed below.
Safety
The safety of nuclear reactors has become paramount since the Fukushima accident of 2011. The lessons learned from that disaster included the need to (1) adopt risk-informed regulation, (2) strengthen management systems so that decisions made in the event of a severe accident are based on safety and not cost or political repercussions, (3) periodically assess new information on risks posed by natural hazards such as earthquakes and associated tsunamis, and (4) take steps to mitigate the possible consequences of a station blackout.
Credit : Britannica
Picture Credit : Google